CSL CENTRO SPERIMENTALE DEL LATTE:
PROBIOTIC MAKERS: FROM DEVELOPMENT TO PRODUCTION
Originally founded in 1948, by Dr. Leo Vesely, in order to study and increase the value of lactic acid bacteria (LAB) and other food grade microorganisms. CSL has a rich history of supporting companies to achieve their Probiotic goals.
CSL was the first Italian company in the study and demonstration of the importance of LAB cultures for human health and wellness.
In 2013, a new chapter in the CSL success story took place, as CSL was acquired by the owners of Caglificio Clerici and Sacco. This purchase allowed CSL to expand further with more than 100 years of experience and history in studying and working with enzymes and cultures from across the group of companies.
Centro Sperimentale del latte, along with Califico Clerici, Sacco and Kemilkalla is a company of Sacco System. The convergence of International Biotech excellence applied to food, nutraceutical and pharmaceutical Industry.
Sacco System’s mission is “Supporting food culture and life.”
Improving the lives of consumers, through good nutrition. Through the know-how of Sacco System’s four companies, good nutrition is delivered in the manufacturing of natural, functional and healthier finished products.
Through Sacco System, CSL has been able to expand and support clients with innovative technologies, from a combined life science centre of excellence developed from group-wide skills and competencies. Today CSL studies, develops and manufactures natural Probiotics for the pharmaceutical, nutraceutical, dairy, food and agro-zoo-technical sectors in 110 countries world wide.
CSL CENTRO SPERIMENTALE DEL LATTE is now bringing 70 years of experience to the Asia Pacific region with CSL Asia Pacific.
CSL ASIA PACIFIC:
OUR SERVICES
CSL Asia Pacific will focus on the
development of the probiotic business in the nutraceutical and pharmaceutical sectors.
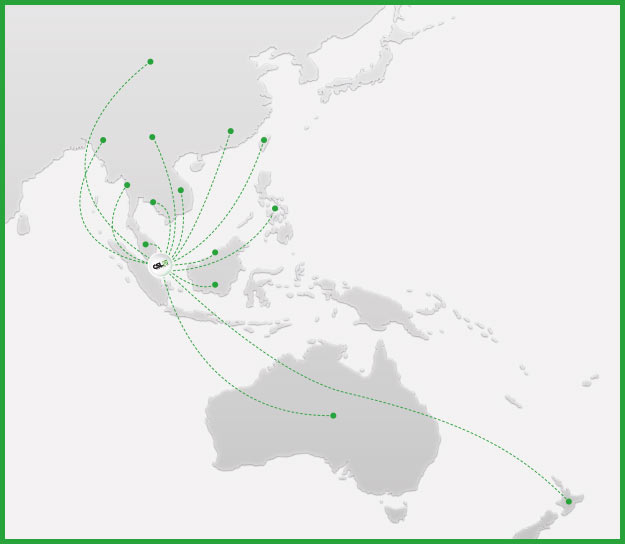
The company will be active in these territories:
Australia
New Zealand
Malaysia
Indonesia
Philippines
Thailand
Vietnam
Cambodia
Laos
Myanmar
Brunei
Hong Kong
Taiwan
China
The company will provide the following services:

High quality & Well documented Probiotics
● Clinically studied strains
● Pre-clinically studied
strains
● Single active & customer
specific probiotic blends

Regulatory Support
● Dossier preparation for
product registration
● Stability programs,
including strenuous
Zone4b regions

Technical Support
● Product conceptualisation
● Custom-made formulations
● Ready to market
formulations
● Technical inquiries

Clinical Trial Support
● In-house laboratories
in Europe and Asia
● Set-up, organise & coordinate
clinical trials in the region
● R&D
● Quality compliance /
certification with ISO
accreditation

Commercialisation Support
● Finished product solutions
● Ready to market product
● Localised marketing
● Product launch training and
conferences in the region
● Market expansion advice

